mRNA-based Rabies Vaccine Makes Progress

Everest Medicines announced yesterday that it had achieved a preclinical (non-human) proof-of-concept milestone for its mRNA rabies vaccine program.
In a head-to-head immunogenicity comparison study with a commercial inactivated rabies vaccine, mice dosed with Everest's preclinical rabies vaccine candidate produced higher levels of serum-neutralizing antibodies than mice dosed with the comparator vaccine.
Everest's candidate also induced a significantly higher degree of T-cell-mediated immune responses.
These positive results support the clinical development of the rabies vaccine, stated the company on December 14, 2022.
Rogers Yongqing Luo, CEO of Everest Medicines, commented in a related press release, "Building on the momentum of promising mRNA infectious disease vaccine candidates, we are well-positioned to continue innovating novel candidates using this mRNA technology platform across a wide range of disease indications."
The Everest vaccine candidate for rabies post-exposure prophylactic was developed in partnership with Providence Therapeutics Holdings Inc. utilizing a clinically validated mRNA technology platform. Everest and Providence hold 50/50 global rights to develop and commercialize the new rabies vaccine.
Everest is a biopharmaceutical company focused on developing and commercializing transformative pharmaceutical products that address critical unmet medical needs for patients in Greater China and other parts of Asia.
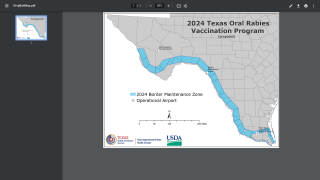
Commercially available rabies vaccines and candidate information are posted at PrecisionVaccinations.com/Rabies.
Our Trust Standards: Medical Advisory Committee