mRNA Vaccine Candidates Combat Malaria
A research team led by George Washington University has developed two mRNA vaccine candidates that are highly effective in reducing malaria infection and transmission.
The team also found that the two experimental vaccines induced a powerful immune response regardless of whether they were given individually or in combination.
“Malaria elimination will not happen overnight, but such vaccines could potentially banish malaria from many parts of the world,” Nirbhay Kumar, a professor of global health at the George Washington University Milken Institute School of Public Health, commented in a press release on December 1, 2022.
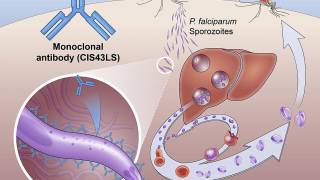
Kumar and the research team focused on the parasite Plasmodium falciparum, one of four parasite species that cause malaria and the deadliest to humans.
Transmitted through the bite of the Anopheles mosquito, P. falciparum, together with P. vivax, are responsible for more than 90% of all malaria cases globally and 95% of all malaria-related fatalities.
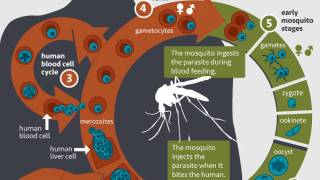
The researchers immunized one group of mice with an mRNA vaccine targeting a protein that helps the parasites move through the body and invade the liver.
They immunized another group of mice with a vaccine targeting a protein that helps parasites reproduce in a mosquito’s midgut. The immunized mice were challenged with the parasite-causing infection, and vaccine-induced antibodies were tested to interrupt malaria transmission.
The study found both vaccines induced a potent immune response in the mice and were highly effective in reducing infection in the host and the mosquito vector.
The presence of protective antibodies during the transmission of parasites to healthy mosquitoes dramatically reduced the parasite load in the mosquitoes, an essential step in disrupting malaria transmission, according to the researchers.
The team also immunized mice with both vaccines and found that co-immunization effectively reduced infection and transmission without compromising the immune response.
To see how the mRNA vaccines stacked up against other nucleic acid-based vaccine platforms, Kumar and the team repeated the experiment using DNA plasmids.
The mRNA vaccines were found to be far superior in inducing an immune response compared to the DNA-based vaccines.
The team hopes to usher the vaccines through additional research, including studies in nonhuman primate models, to produce vaccines that can be used safely in humans.
“To have a vaccine cocktail that can effectively disrupt multiple parts of the malaria parasite’s life cycle is one of the holy grails of malaria vaccine development,” Kumar said.
“This study brings us one step closer to producing vaccines that can be used safely in humans to prevent illness and save lives–with the ultimate goal of defeating this disease.”
The U.S. NIH supported the study. The team, which has filed for a patent, developed the vaccines in partnership with scientists from the University of Pennsylvania and other collaborators.
The study was published in npj Vaccines.
As of December 1, 2022, the U.S. FDA had not approved a malaria vaccine. However, the FDA did approve Artesunate to treat severe malaria in adult and pediatric patients in May 2020.
Additional malaria information is posted at Vax-Before-Travel.com.
Our Trust Standards: Medical Advisory Committee