Ebola Vaccine Candidates Selected for Human Study
The World Health Organization recently announced three Sudan Ebolavirus (SUDV) vaccine candidates had been selected for testing in the Republic of Uganda.
As of November 25, 2022, no U.S. FDA-approved SUDV vaccines are available.
Three candidate vaccines are as follows:
A bivalent adenovirus vectored vaccine (biEBOV) which consists of the replication-deficient simian adenovirus vector ChAdOx1 encoding two antigens: EBOV glycoprotein (Zaire) and SUDV glycoprotein (Sudan) developed by the University of Oxford and the Jenner Institute UK.
A monovalent adenovirus vectored vaccine consists of the simian adenovirus vector ChAd3 encoding the SUDV glycoprotein (ChAd3-SUDV) and is produced by the Sabin Vaccine Institute USA.
A monovalent vaccine that consists of the vesicular stomatitis virus as the backbone with the VSV-G gene replaced with the Ebola-GP gene from the Sudan strain (VSV-SUDV) from the International Aids Vaccine Initiative.
These vaccines will begin human clinical trials in 2022.
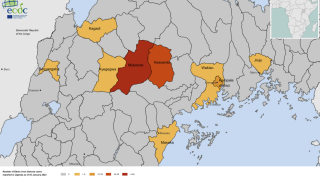
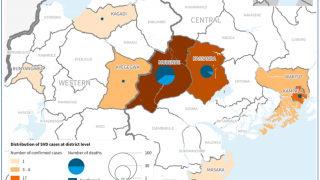
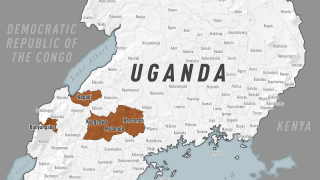
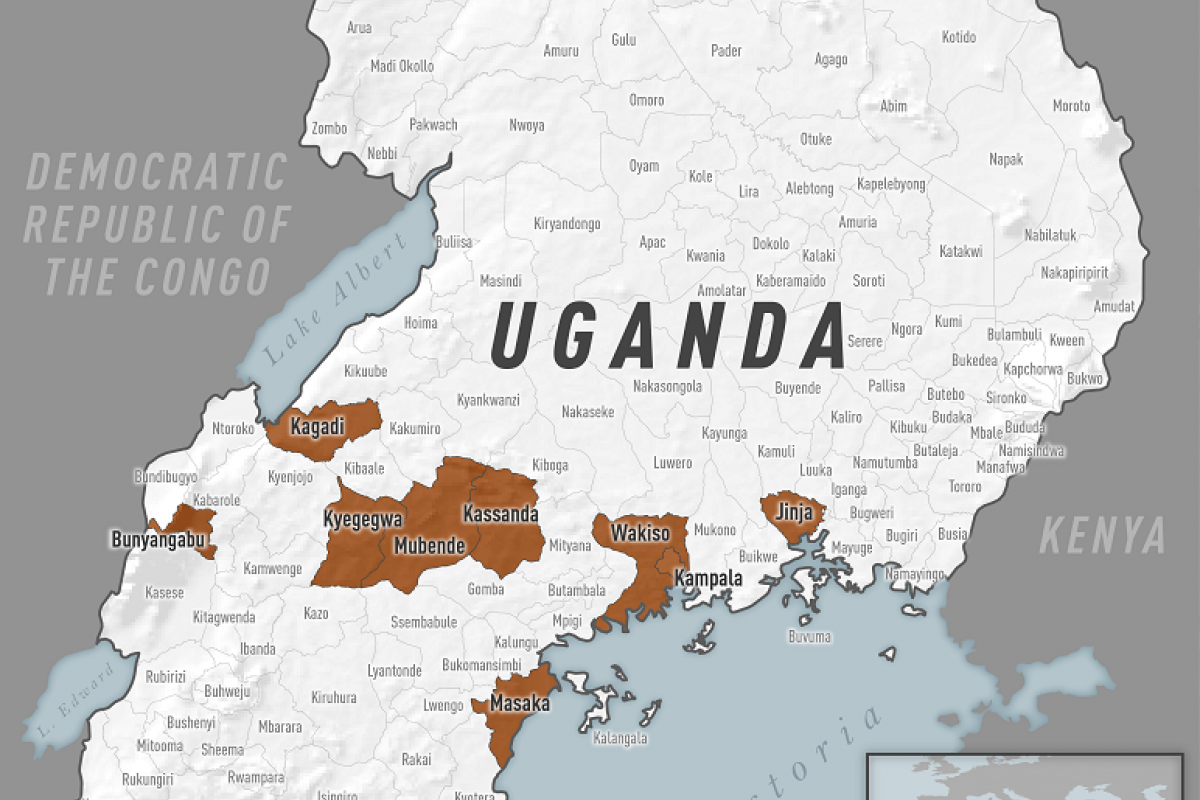
Since the outbreak declaration on September 20, 2022, a total of 141 confirmed cases and 55 confirmed deaths (CFR 39%) from Ebola disease caused by the SUDV have been reported.
Overall, 19 cases with seven deaths occurred among healthcare workers.
To notify travelers of their potential health risks during this Ebola outbreak, the U.S. CDC reissued an Alert - Level 2, Practice Enhanced Precautions, on November 15, 2022.
Additional SUDV vaccine development news is posted at PrecisionVaccinations.com/Ebola.
Our Trust Standards: Medical Advisory Committee