Adjuvanted Malaria Vaccine Continues Protecting Children

The Lancet Infectious Disease previously reported the efficacy of the R21/Matrix-M malaria vaccine, reaching the World Health Organization (WHO) goal of 75% or greater efficacy over 12 months in the target African children.
In this early release report on December 1, 2022, researchers confirmed the safety, immunogenicity, and efficacy results at 12 months following the administration of a booster vaccination.
This double-blind phase 1/2b randomized controlled clinical trial was done in toddlers aged 5–17 months in Nanoro, Burkina Faso.
Eligible children were enrolled and randomly assigned (1:1:1) to receive three vaccinations of either 5 μg R21/25 μg Matrix-M, 5 μg R21/50 μg Matrix-M, or a control vaccine (the Rabivax-S rabies vaccine) before the malaria season, with a booster dose administered 12 months later.
The study's results showed a booster dose of R21/Matrix-M at one year following the primary three-dose regimen maintained high efficacy against first and multiple episodes of clinical malaria.
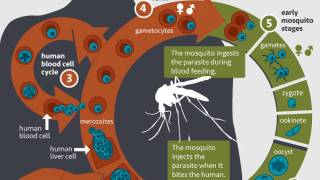
According to the U.S. CDC, malaria is a vaccine-preventable mosquito-borne disease.
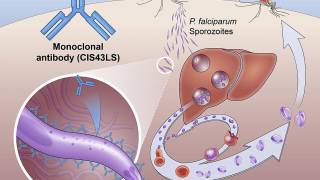
R21 and the RTS,S/AS01 vaccine both have a similar immunogen that targets Plasmodium falciparum, the parasite that causes malaria in humans.
However, R21 is adjuvanted with Matrix-M to enhance the immune response.
Additional malaria vaccine information is posted at Vax-Before-Travel.com/Malaria.
Disclosures: This clinical trial was mainly funded by a European and Developing Countries Clinical Trials Partnership grant to the Multi-Stage Malaria Vaccine Consortium, with additional support from the Wellcome Trust through Translation Award, and from the U.K. National Institute for Health Research to the Oxford Biomedical Research Centre's Vaccines for Emerging and Endemic Diseases theme.
The vaccine manufacture and supply were supported and undertaken by the Serum Institute of India, and Novavax provided the Matrix-M adjuvant. And authors declare competing interests.
Our Trust Standards: Medical Advisory Committee