Alzheimer's Disease Therapeutic Vaccine Candidate Study Continues in Finland
Gothenburg-based Alzinova AB announced today that a second external safety review had been completed with a positive assessment to continue the phase 1b clinical study with ALZ-101 in patients with early Alzheimer's disease (AD).
The study investigates two different dose strengths of ALZ-101 during a treatment period of 20 weeks. In total, 26 study participants based in Finland will receive four doses of either ALZ-101 or a placebo.
Alzinova's approach is to develop a therapeutic vaccine that specifically targets the toxic accumulations of amyloid-beta in the form of oligomers in the brain, which has several advantages compared to other methods.
This AD vaccine candidate is designed to provoke an immune response specific to soluble oligomeric Aβ assemblies but not monomeric or fibrillar Aβ.
A proprietary protein-engineering technology uses disulfide bonds to cross-link Aβ peptides into a conformation that assembles into stable, soluble oligomers or protofibrils. These assemblies are formulated into the ALZ-101 vaccine.
This means people vaccinated with ALZ-101 generate their antibodies, specifically against toxic accumulations of amyloid-beta oligomers in the brain.
Kristina Torfgård, CEO at Alzinova, commented in a press release on September 7, 2022, "We are happy to have received continued positive feedback from the independent expert group and that the study shows that the treatment is well tolerated with no signs of that it is harmful."
"Furthermore, the vaccine is prepared to be produced in larger volumes for phase 2 development. "
"We are convinced that we will make it possible for Alzheimer's patients to live an independent and active life."
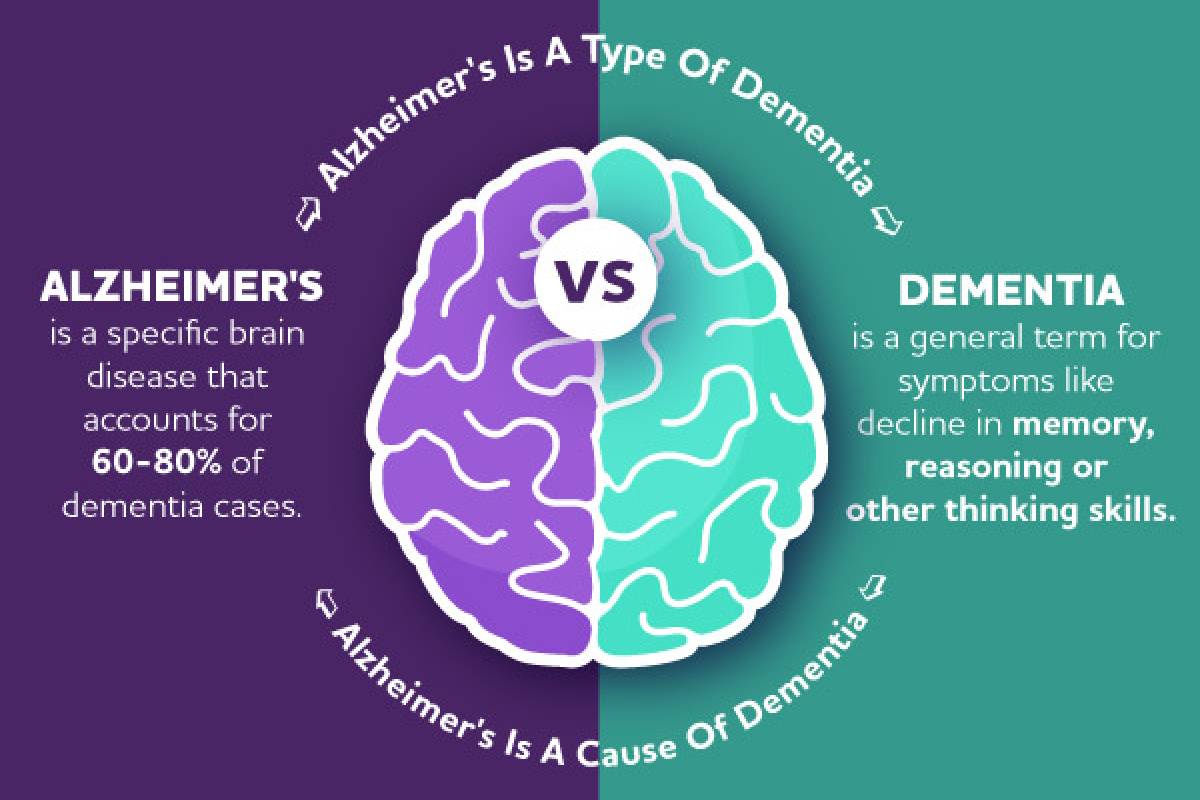
According to the Alzheimer's Association, AD is a degenerative brain disease caused by complex brain changes following cell damage, leading to dementia.
Dementia is a general term for a decline in mental ability severe enough to interfere with daily life.
Many different types of dementia exist, and many conditions cause it.
As of September 7, 2022, the U.S. FDA had not approved an AD vaccine.
However, there are several Alzheimer's vaccine candidates conducting clinical studies.
Note: This announcement was manually translated and curated for mobile readership.
Our Trust Standards: Medical Advisory Committee














