Modified Bladder Cancer Vaccine Shows Early-Stage Promise
A modified tuberculosis vaccine (BCG) developed in San Antonio by Texas Biomed could help treat a form of bladder cancer called non-muscle invasive bladder cancer (NMIBC).
Published online in the journal Cancer Immunology, Immunotherapy on June 24, 2022, this non-human research used mouse models and human cells to evaluate the efficacy and immune impact of selective delipidation in dBCG-Tokyo and dBCG-TICE strains.
Both dBCG-Tokyo and dBCG-TICE effectively treated subcutaneous MB49 tumors in mice and enhanced tumor-infiltrating CD8+ T and natural killer cells, similar to conventional BCG vaccination.
However, compared to conventional BCG, only dBCG-Tokyo retained a significant effect on intratumoral tumor-specific CD8+ and γδ T cells by increasing their frequencies in tumor tissue and their production of antitumoral function-related cytokines, i.e., IFN-γ and granzyme B.
Further, dBCG-Tokyo enhanced the function and cytotoxicity of innate effector cells against human bladder cancer T24 in vitro.
“I’m hopeful that with grant or industry support, we can move this right along to clinical trials and explore this treatment for patients who don’t have options other than bladder removal,” stated Robert S. Svatek, MD, a urologic oncologist who treats bladder cancer patients at the Mays Cancer Center at UT Health San Antonio, Texas, and is co-senior author of the research paper.
Dr. Svatek commented in a press release issued on August 22, 2022, “We’re talking about a very select group of patients, but for whom this matters.”
This is essential research since about 61,700 men and 19,480 women each year get bladder cancer, and about 17,100 deaths are due to the disease.
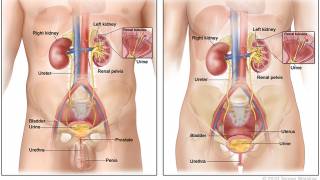
About 75% of bladder cancer cases are classified as non-muscle invasive, which means the cancer affects the tissue lining the inner surface of the bladder but not the bladder muscle.
Texas Biomed Professor Jordi B. Torrelles, Ph.D., specializes in tuberculosis and has been working on a modified BCG vaccine for the past six years to improve TB treatment in the lungs, removing specific lipids from the cell envelopes of the bacteria in the BCG vaccine.
This “delipidated” vaccine still triggers the body to produce well-regulated immune responses but reduces overzealous inflammation that causes severe tissue damage.
“It is more targeted and allows for a longer, slower response, which makes it more effective,” Dr. Torrelles says.
“We are excited to see this move forward, and since it is based on an already U.S. FDA-approved treatment, we are hopeful it can proceed in a timely way,” Dr. Torrelles says.
The Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccine was developed for tuberculosis in the 1920s and has been used to treat non-muscle invasive bladder cancer since the late 1970s by stimulating an immune response at the cancer site.
It was one of the first cancer immunotherapies and is more effective than chemotherapy for this type of cancer – but up to 84% of patients cannot tolerate the strong side effects and don’t complete the three years of BCG treatment.
When treatments fail, the last option is removing the bladder, which reduces the quality of life.
Additional BCG vaccine therapy uses are posted at PrecisionVaccinations.com/BCG.
Our Trust Standards: Medical Advisory Committee