VLP Vaccine Candidate for the Prevention of Acute Gastroenteritis Caused by Norovirus Launches Phase 2b Study with Children
Massachusetts-based HilleVax, Inc. reported today dosing the first subjects of 3,000 in its Phase 2b clinical trial of HIL-214 in the U.S. and Latin America.
HIL-214 is a virus-like particle, 2-dose, bivalent vaccine candidate to prevent moderate-to-severe acute gastroenteritis (AGE) caused by norovirus infection in infants.
There are currently no U.S. FDA-approved vaccines or antiviral therapies for either the prevention or treatment of norovirus-related illness.
The trial's primary objective is to evaluate the protective efficacy of HIL-214 against the first confirmed moderate or severe AGE event due to GI.1 or GII.4 norovirus strains (excluding certain co-infections) that occur before each subject reaches one year of age.
“We’re pleased to have dosed the first subjects in this Phase 2b study, the first-ever field efficacy clinical trial of a norovirus vaccine candidate in infants,” said Rob Hershberg, MD, Ph.D., Chairman and CEO of HilleVax, in a press release issued on May 2, 2022.
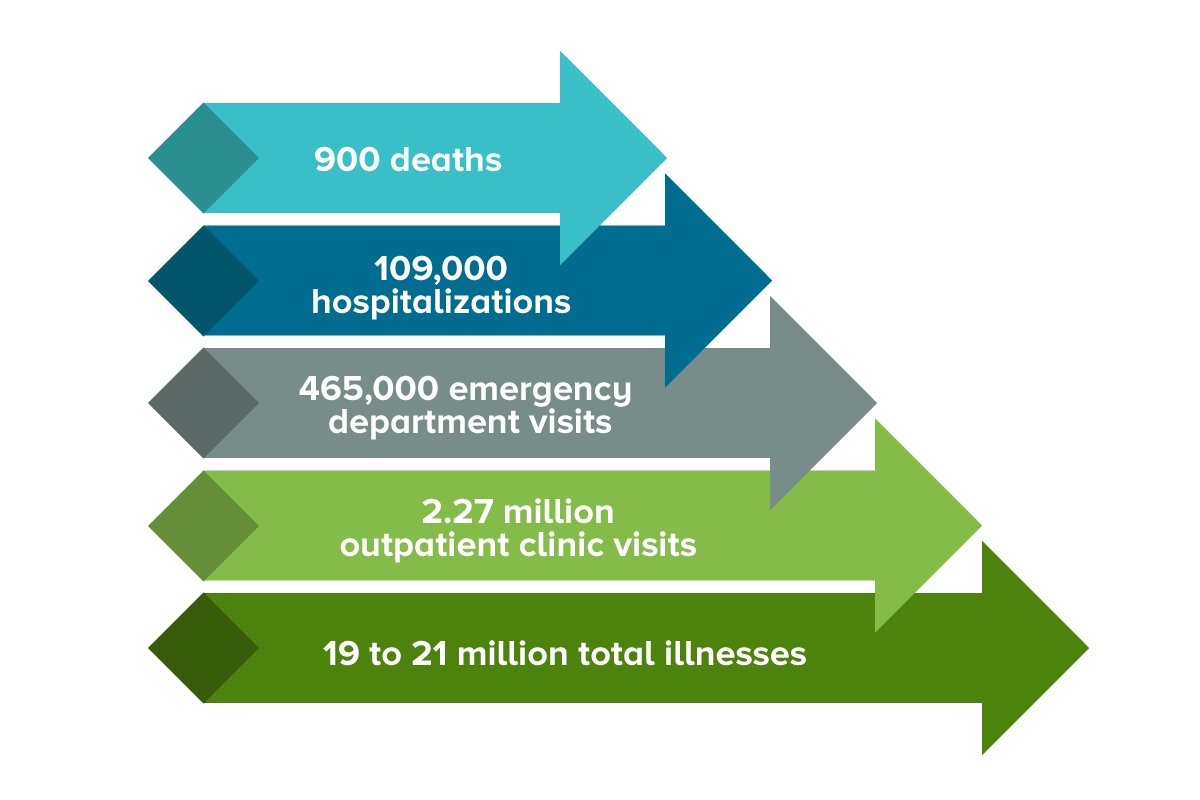
“We believe HIL-214 has the potential to address a vast unmet need which includes approximately 700 million cases, 200,000 deaths, and $60 billion of economic burden from norovirus-related AGE worldwide each year.”
HIL-214 has been studied in nine clinical trials, which collectively generated safety data from more than 4,500 subjects and immunogenicity data from more than 2,200 subjects, including safety and immunogenicity data from more than 800 pediatric patients subjects.
According to the U.S. CDC, norovirus is the most common cause of viral AGE worldwide.
Children and older adults are more likely to have an outpatient or emergency department visit than people of other ages.
It is characterized by symptoms including diarrhea, vomiting, abdominal pain, nausea, and, sometimes, fever that may lead to clinically significant dehydration.
Most norovirus outbreaks in the U.S. happen from November to April.
In years when there is a new strain of the virus, there can be 50% more norovirus illness.
Note: The company's press statement was edited for clarity and manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee