Monkeypox in Texas, What Happened

The U.S. CDC's Morbidity and Mortality Weekly Report published today reviewed the monkeypox virus cases confirmed in a U.S. resident who had returned to Dallas, Texas, from Nigeria in July 2021.
The Dallas County Health and Human Services Laboratory Response Network laboratory-confirmed, by real-time polymerase chain reaction, the presence of nonvariola orthopoxvirus DNA from this Nigerian traveler from lesion swabs, confirming the West African clade Monkeypox virus.
The patient received tecovirimat, an antiviral for treating orthopoxvirus infections and recovered.
Since 2017, this was the first travel-associated monkeypox case in the U.S. and the seventh such case globally.
Monkeypox is a rare, sometimes life-threatening zoonotic infection and can spread to humans.
It is caused by the Monkeypox virus, an orthopoxvirus similar to Variola virus (the causative agent of smallpox) and Vaccinia virus (the live virus component of orthopoxvirus vaccines).
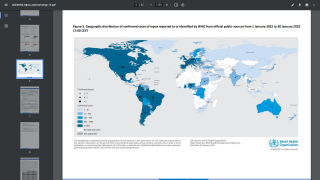
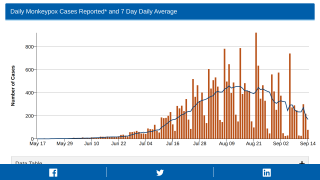
The CDC and local public health authorities launched an intensive investigation from July 13 to September 4, 2021.
Among 194 monitored contacts in the Dallas area, 144 (74%) were flight contacts.
Whole-genome sequencing showed that the virus was consistent with a strain of Monkeypox virus known to circulate in Nigeria. Still, the specific source of the patient's infection was not identified.
And no contact received post-exposure prophylaxis with the orthopoxvirus vaccine ACAM2000®, a smallpox vaccine consisting of a live, infectious vaccinia virus.
Four months after this case in Texas, an eighth travel-associated monkeypox case in a traveler from Nigeria occurred, also in the U.S., prompting CDC to issue a Level 1 Travel Health Notice for travel to Nigeria.
Multiple reasons have been proposed for continued human cases in Nigeria, including population growth, increased human interaction with Monkeypox virus reservoirs because of deforestation and climate change, accumulation of unvaccinated cohorts, and declining smallpox vaccine immunity.
The Nigerian Federal Ministry of Health continues to work to prevent, detect, and investigate monkeypox cases in Nigeria, but as patients continue to occur, U.S. public health and hospital authorities might consider developing local strategies for responding to future imported cases.
Understanding the types of exposures that are most concerning for Monkeypox virus transmission, knowing contaminated surfaces need to be quickly identified and decontaminated, and anticipating potentially lengthy hospitalizations and contact monitoring periods might also aid in planning for future cases.
In preparation for a monkeypox / smallpox outbreak in the U.S., the Department of Health and Human Services awarded a contract to Maryland-based Emergent BioSolutions Inc. in September 2019 valued at approximately $2 billion over ten years to supply ACAM2000 to the U.S. Strategic National Stockpile.
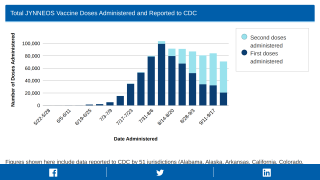
A newer vaccine, JYNNEOS (IMVANEX, IMVAMUNE), was Approved by the U.S. FDA in 2019 and is indicated for preventing smallpox and monkeypox disease in adults.
Bavarian Nordic's JYNNEOS is the only FDA-approved non-replicating smallpox vaccine and the only monkeypox vaccine for non-military use.
Note: This news article edited the CDC report for clarity and was manually curated for mobile readership
Our Trust Standards: Medical Advisory Committee