Yellow Fever Vaccine Booster Candidate Advances

U.K.-based Emergex Vaccines Holding Limited today announces that it has agreed with ATCC to progress preclinical development of its Yellow Fever booster vaccine program.
The agreement with ATCC is expected to enable Emergex to perform its studies safely with the wildtype Yellow Fever virus, classified as a Biosafety Level 3 pathogen, to advance the development of its T-Cell booster vaccine candidate.
ATCC will oversee and perform viral infection models – infecting human cell lines with wildtype Yellow Fever virus. Emergex will then conduct immunoproteomics analyses of the MHC Class I-presented viral peptides on infected cell surfaces to confirm the expression library of viral epitope peptides presented to CD8+ T-Cells.
The anticipated results, together with earlier data derived from the live attenuated virus, are expected to allow for the development of an effective next-generation Yellow Fever vaccine capable of meeting an increasing global vaccine demand.
Professor Thomas Rademacher, co-founder, and CEO of Emergex Vaccines, commented in a press statement issued on April 7, 2022, "Emergex is making progress in advancing a T-Cell Adaptive booster vaccine candidate through its confirmatory ligandome studies using wildtype Yellow Fever virus may be a promising solution for ongoing protection as this infectious disease continues to re-emerge and spread worldwide."
"While the current YF17D vaccine (Stamaril) is considered effective, reliance on immunization as primary protection against the disease is potentially unsustainable in the long-term."
"This conclusion is important because many individuals in endemic regions with primary Yellow Fever immunization may require a booster to ensure continued protection, thus exacerbating existing vaccine shortages."
"Investment in next-generation Yellow Fever primary vaccines and boosters is therefore critical to providing additional supply of treatments to meet the increasing global demand and minimize the side effects of YF17D."
In August 2020, researchers at Emergex reported the first analysis of T-Cell epitopes produced by an existing live attenuated commercial Yellow Fever 17D (YF17D) vaccine.
Emergex now plans to perform a comparative study using the wildtype Yellow Fever virus: these comparison studies of the Class I CD8+ T-cell epitopes derived from both live attenuated and wildtype viruses will further support the development of Emergex's T-Cell Yellow Fever booster vaccine candidate.
ATCC is a non-profit organization that collects, stores, and distributes standard reference microorganisms, cell lines, and other materials for research and development.
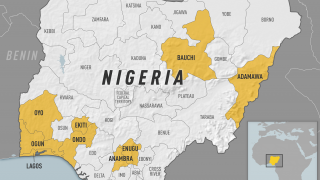
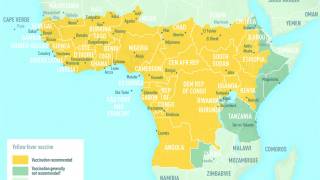
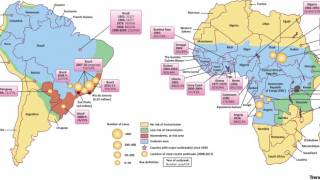
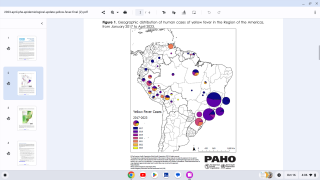
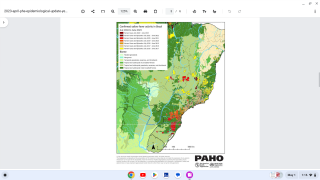
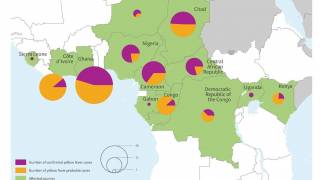
Yellow Fever is a severe, potentially fatal, mosquito-borne disease. There are 47 at-risk countries causing concern for future disease outbreaks.
Previous yellow fever vaccine news is posted at Vax-Before-Travel.com.
Note: The company's press release was edited for clarity and manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee