Needle-Free COVID-19 Vaccine Candidate Enters Clinical Trial
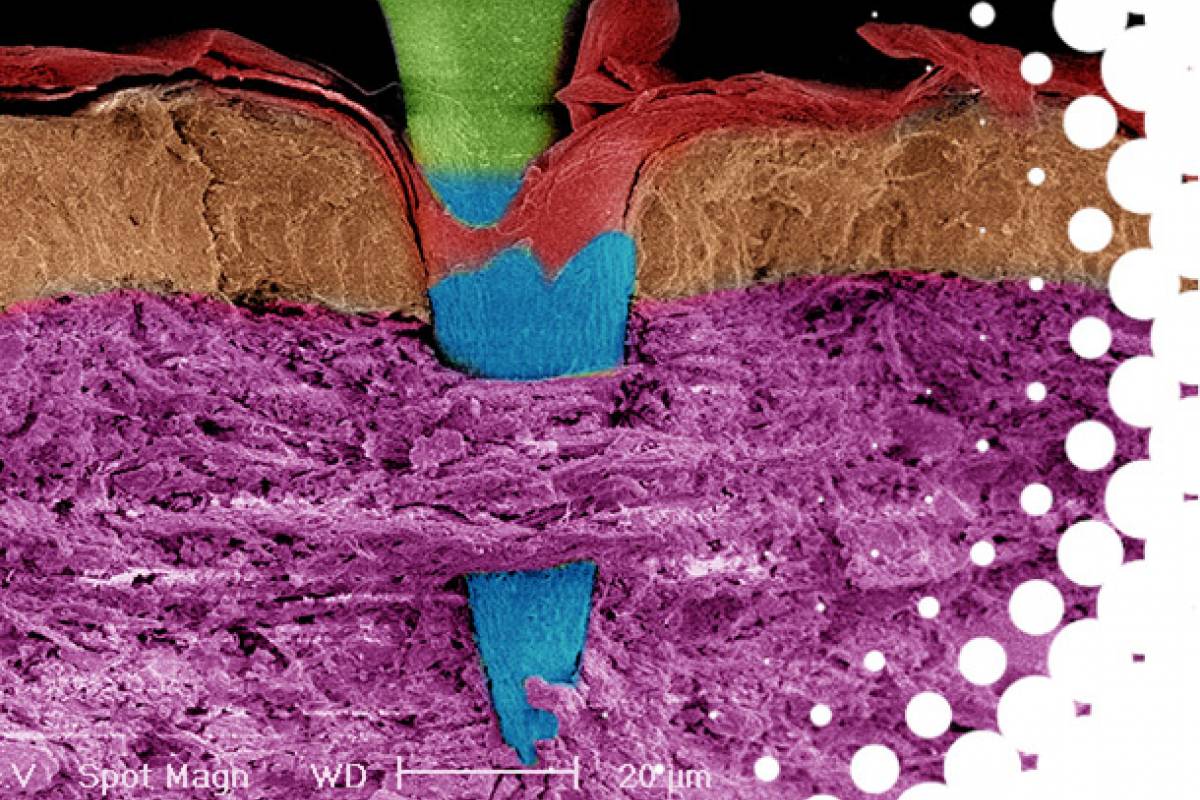
Massachusetts - based Vaxxas today announced that The University of Texas (UT) at Austin had granted an exclusive license to a next-generation SARS-CoV-2 spike subunit vaccine (HexaPro) for vaccination using a patch.
The license from UT Austin, along with a companion background technology license obtained by Vaxxas from the U.S. National Institutes of Health, enables Vaxxas to create the first needle-free, room-temperature stable COVID-19 vaccine patch to enter clinical studies.
Vaxxas intends to complete Phase 1 clinical studies in 2022 with HexaPro to be delivered using Company's proprietary microarray patch (HD-MAP) technology.
HexaPro is a highly stabilized protein designed to mimic the structure of the spike protein on the surface of the coronavirus to train the human immune system to recognize and fight a SARS-CoV-2 infection.
HexaPro is the most advanced spike protein from UT Austin's world-renowned vaccine development team.
Preclinical research published in 2021 by Science Advances demonstrated that HexaPro delivered using Vaxxas' HD-MAP (HD-MAP/HexaPro) resulted in enhanced virus-neutralizing antibody and T-cell responses against all variants of concern, including alpha, beta, gamma, delta, and omicron, when compared to needle and syringe vaccination with HexaPro.
Vaxxas has completed three human clinical studies of HD-MAP involving more than 300 participants, demonstrating safety and enhanced immune response of vaccine administration by HD-MAP.
"Just as the virus has changed and evolved, our vaccines need to keep up with the latest challenges, too, and a key challenge now is vaccinating the world," said Dr. Jason S. McLellan, Professor of Molecular Biosciences and Robert A. Welch Chair in Chemistry at the University of Texas at Austin, in a press release issued on March 23, 2022.
"It's exciting to see HexaPro on the cusp of entering clinical trials with a patch-based technology that could be a real game-changer for parts of the globe where access to vaccines has been limited so far."
The UT at Austin's McLellan and his collaborators at the NIH are the designers of an early version of the SARS-CoV-2 spike protein that was genetically altered by swapping in two amino acids called prolines, used to stabilize the spike protein and allow the immune system to better fight off infection.
The earlier spike protein is used in leading first-generation COVID-19 vaccines, including all vaccines currently distributed in the U.S. and others approved under emergency use authorization in multiple countries.
Note: This news article edited a press release for clarity and was manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee























