HPV Vaccination Not Associated with Primary Ovarian Insufficiency

A large study of about 1 million women in Denmark found no association between 4HPV vaccination and primary ovarian insufficiency.
The JAMA Network published an Original Investigation in late August 2021 that concluded by saying this 'study provides much-needed support for the ovarian safety of the 4HPV vaccine.
This finding is essential for clinical and public health personnel when addressing parental concerns about Human Papillomavirus (HPV) vaccination-associated fertility issues.
There is currently little evidence for an association between 4HPV vaccination and primary ovarian insufficiency. On the contrary, the vaccine protects against infections that may decrease fertility.
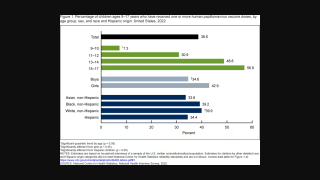
This retrospective analysis followed 996,300 Danish women from 2007 to 2016 between the ages 11 to 34 years.
About half of the women (n = 505,829) had received 4 HPV vaccine doses, while the other half (n = 490,471) were unvaccinated.
Overall, 144 women were diagnosed with primary ovarian insufficiency, 54 of which had received four doses of the HPV vaccination.
Moreover, the researchers determined that 26.94 years was the median age of diagnosis.
Previously, a study published in July 2020 completed a disproportionality analysis utilizing the Vaccine Adverse Event Reporting System database and reported safety signals associated with 4HPV and menstruation irregularities.
However, while safety signals generated from passive surveillance contribute little to the assessment of causality, they may warrant further investigation in analytical studies.
Although 4HPV was not associated with a composite outcome of oligomenorrhea and amenorrhea in this new study, the results suggest a statistically insignificant increase in the rate of this outcome.
'Our findings suggest that the increase is transient and is not associated with primary ovarian insufficiency diagnoses,' concluded these researchers.
There are safe and effective HPV vaccines to protect males and females against cancers caused by HPV.
These vaccines include 9vHPV, 4vHPV, and/or 2vHPV, says the US Centers for Disease Control and Prevention (CDC). HPV vaccination for adolescents has been recommended for females since 2006 and males since 2011.
The current HPV vaccine recommendations are found on this CDC website. In addition, for the full text of CDC's Advisory Committee on Immunization Practices recommendations, see the Human Papillomavirus Vaccine Recommendations.
Our Trust Standards: Medical Advisory Committee