Organ Transplant Patients Join mRNA COVID-19 Vaccine Study
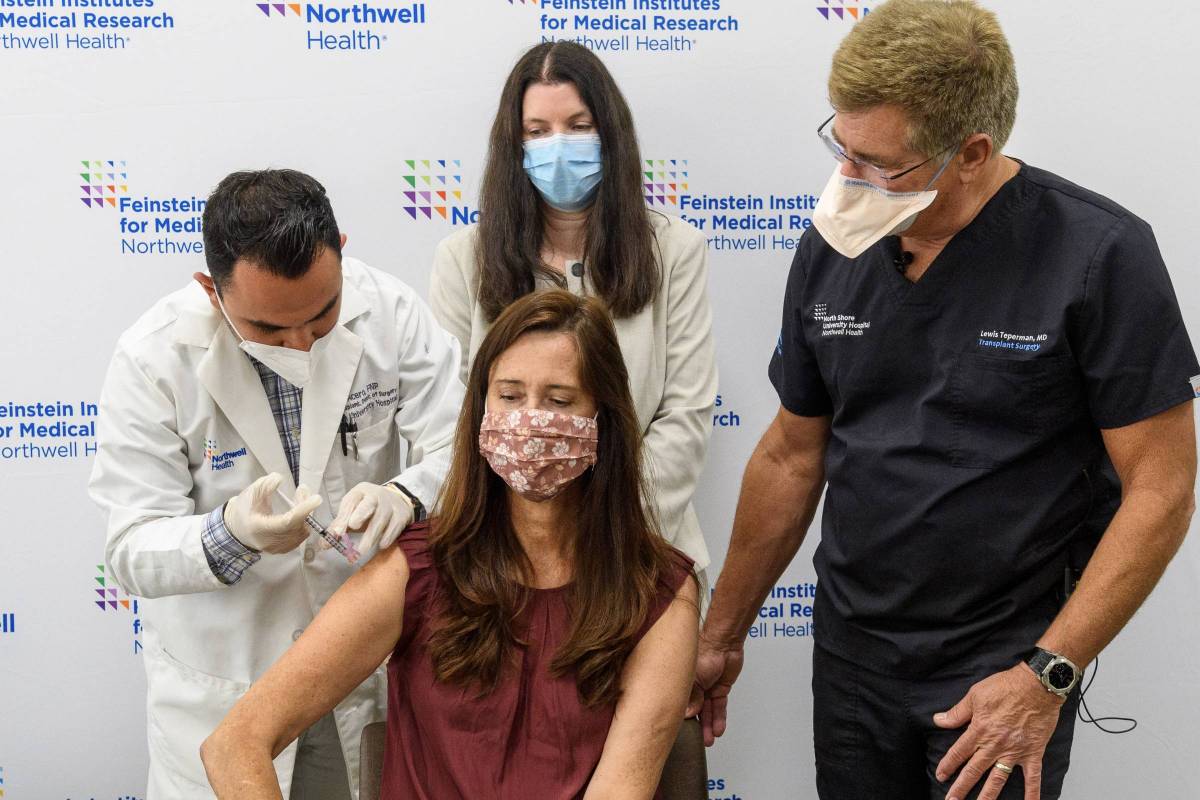
People who have received an organ transplant are at high risk for contracting COVID-19 and becoming severely sick, stated a press release issued by the Feinstein Institutes for Medical Research.
And after receiving a transplanted organ, immunosuppressive drugs may interfere with the recipient’s ability to build a strong immune defense against the SARS-CoV-2 betacoronavirus from the first doses of a COVID-19 vaccine.
A new multi-centered, nationwide phase 3 clinical trial is looking at the efficacy of a third Moderna SpikeVax vaccine dose for COVID-19 in people living with an organ (liver or kidney) transplant.
The Feinstein Institutes administered an extra vaccine to the first set of patients in the United States on September 20, 2021. This trial will dose patients with an extra shot and monitor their immune antibody response 28 days from the third shot.
“Organ transplant recipients may not have as strong of an immune response to the COVID-19 vaccines as the general population does, leaving them vulnerable to the virus,” said Lewis Teperman, M.D., director of transplant services for Northwell Health and principal investigator on the trial, in a related press release.
“We are eager to provide patients more vaccine to help protect them and to gain much-needed scientific evidence to help doctors best treat their patients.”
People who have received a solid organ transplant within the last six months may qualify for the trial. Moderna is hoping 240 participants (including healthy volunteers) will be enrolled in the trial nationwide. The Feinstein Institutes is one of the seven sites leading the trial and the first to enroll participants.
In March 2020, the Feinstein Institutes announced its first clinical trials focused on studying the safety and efficacy of potential COVID-19 therapies. Since then, Feinstein Institutes initiated more than 17 clinical trials and programs and enrolled more than 1,800 patients.
Most recently, the Feinstein Institutes began to enroll patients in another national clinical trial that delivers extra vaccinations to patients with an autoimmune disease. Researchers will investigate whether antibody response to the vaccine is related to medications, disease, and/or vaccine type.
Long Island-based Feinstein Institutes for Medical Research is the research arm of Northwell Health, the largest health care provider and private employer in New York State.
Note: August 25, 2021, Moderna, Inc. announced it had completed the rolling submission process for its Biologics License Application to the U.S. FDA for the full licensure of the SpikeVax COVID-19 Vaccine for active immunization to prevent COVID-19 in individuals 18 years of age and older.
Our Trust Standards: Medical Advisory Committee























