Sao Paulo Offering Hep A Vaccinations to Young Men

An outbreak of Hepatitis A in Sao Paulo, Brazil has already surpassed the number of cases reported in 2016 by 700%.
According to data released by the Municipal Health Secretariat, between January 1 and September 16 of 2017, Sao Paulo has reported 517 cases of hepatitis A.
During 2017, 2 fatalities have been reported in connection with this Sao Paulo Hepatitis outbreak.
According to Flavia Jacqueline Almeida, an infectious pediatrician and an assistant professor in the Pediatric Department of the Faculty of Medical Sciences of Santa Casa de São Paulo, 87% of these Hepatitis A notifications - 452 cases - have occurred among male patients aged between 18 and 39 years.
Dr. Flavis said the most worrying hepatitis A cases are those that progress to so-called fulminant hepatitis A, when the inflammation of the liver is so intense that it leads to the need for urgent liver transplantation and, in extreme situations, to death.
"In São Paulo, four cases of fulminant hepatitis were recorded this year, with two deaths, "says Flavia.
Dr. Flavia points out that the outbreak had already been observed in several European countries since 2016, and the main population affected on the continent were also young, homosexual men.
Other forms of transmission of the hepatitis disease include contact with contaminated food and water.
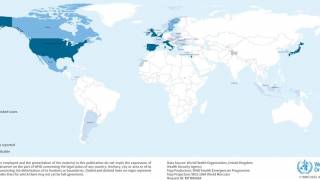
According to the World Health Organization, (WHO), between June 2016 and May 2017, an unusual increase in cases of hepatitis A affecting mainly men who have sex with men (MSM) has been reported by low endemicity countries in the European Region, and in the Americas (Chile and the United States of America).
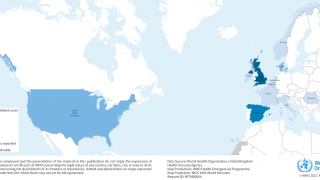
In the European Region, 15 countries (Austria, Belgium, Denmark, Finland, France, Germany, Ireland, Italy, the Netherlands, Norway, Portugal, Slovenia, Spain, Sweden, and the United Kingdom) reported 1,173 cases related to the three distinct multi-country hepatitis A outbreaks as of May 2017.
The WHO recommends hepatitis A vaccination for high-risk groups, such as travellers to endemic areas, MSM, people who inject drugs, and chronic liver disease patients.
The virus is primarily spread when an uninfected (and unvaccinated) person ingests food or water that is contaminated with the feces of an infected person.
The WHO reports hepatitis A has the potential to spread to the general population if control measures (vaccination, hygiene, food safety, and safer sex measures) are not implemented.
The WHO suggests:
- Use of hepatitis A vaccine should be preferred for pre- and post-exposure prophylaxis (e.g. for close contacts of acute cases of hepatitis A).
- Countries may consider single-dose schedule for hepatitis A vaccination when vaccine availability is scarce.
- Public health messaging should be directed at groups at increased risk of hepatitis A and of serious complications from the infection.
In the USA, there are three FDA approved monovalent hepatitis A vaccines:
- Vaqta (Merck) and Havrix (GlaxoSmithKline Beecham Biologicals), are approved for people ≥12 months of age in a 2-dose series
- A combined hepatitis A and hepatitis B Twinrix, (GlaxoSmithKline) vaccine is approved for people ≥18 years of age in the United States
The CDC Vaccine Price List provides current HAV vaccine contract prices and general information.
Vaccine discounts can be found here.
Our Trust Standards: Medical Advisory Committee
- The city registered 517 cases of the disease until October, against 64 in all 2016
- Hepatitis A outbreaks mostly affecting men who have sex with men – European Region and the Americas
- Dr. Cláudio Roberto Gonsalez
- Fulminant hepatic failure: etiology and indications for liver transplantation
- Hepatitis A Questions and Answers for Health Professionals