Meningococcal Vaccines and Soliris Don’t Mix

The risk for meningococcal disease remains high in patients using the blood disorder drug Soliris.
This risk remains high even after they have received a meningococcal vaccine, according to the Centers For Disease Control and Prevention (CDC) research.
But, the CDC says patients should still receive meningococcal vaccines before they begin treatment with Soliris.
“Health care providers should continue to follow recommendations from the Advisory Committee on Immunization Practices for eculizumab (Soliris) recipients to receive both MenACWY and MenB vaccines and could consider antimicrobial prophylaxis for the duration of eculizumab treatment to potentially reduce the risk for meningococcal disease,” Lucy A. McNamara, PhD, of the CDC’s National Center for Immunization and Respiratory Diseases, Division of Bacterial Diseases, and colleagues wrote.
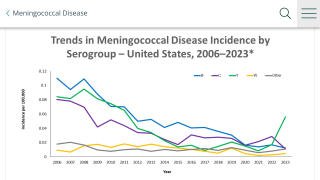
In this CDC study, McNamara and colleagues found 16 cases of meningococcal disease in patients who received eculizumab in the United States from 2008 to 2016.
Fourteen of the 16 patients had documented receipt of at least one dose of the quadrivalent meningococcal conjugate (MenACWY) vaccine, and three of the four cases diagnosed after the CDC recommendation that eculizumab recipients receive both MenACWY and the serogroup B (MenB) meningococcal vaccines also had received one or more doses of MenB before disease onset.
“However, these symptoms can progress to severe illness and death within hours,” the researchers warned.
“Healthcare providers should have a high index of suspicion for meningococcal disease in patients taking eculizumab who develop any symptoms consistent with either meningitis or meningococcemia, even if the patient’s symptoms initially appear mild, and even if the patient has been fully vaccinated or is receiving antimicrobial prophylaxis.”
There are three types of meningococcal vaccines available in the United States:
- Meningococcal conjugate vaccines (Menactra®, Menveo®, and MenHibrix®)
- Meningococcal polysaccharide vaccine (Menomune®)
- Serogroup B meningococcal vaccines (Bexsero® and Trumenba®)
All 11 to 12 year olds should be vaccinated with a meningococcal conjugate vaccine (Menactra® or Menveo®). A booster dose is recommended at age 16 years. Teens and young adults (16 through 23 year olds) also may be vaccinated with a serogroup B meningococcal vaccine.
In certain situations, other children and adults could be recommended to get any of the three kinds of meningococcal vaccines.
The CDC Vaccine Price Lists for general information can be found at this link.
The CDC is asking all state health departments to fill out a report form for all meningococcal disease cases in patients receiving Soliris (eculizumab). That form can be found here. The agency asks that forms be sent by secure email to [email protected] or secure fax at 404-471-8372.
Disclosure: The researchers report no relevant financial disclosures.
Our Trust Standards: Medical Advisory Committee