How Many Doses of Mpox Vaccine Are Needed

The results from a literature search published on April 26, 2024, identified 16 study records that fit the inclusion criteria for reviewing the effectiveness of the JYNNEOS® (MVA-BN) vaccine against mpox.
Where the study population was exclusively or primarily those receiving pre-exposure prophylactic vaccination, JYNNEOS's adjusted vaccine effectiveness (VE) estimates ranged from 35% to 86% (n=8 studies) for one dose.
JYNNEOS's VE for two doses ranged from 66% to 90% (n=5) for two doses.
Where only post-exposure prophylactic vaccination was assessed, adjusted VE estimates were reported for one dose only at 78% and 89% (n=2).
These researchers concluded that 'despite heterogeneity in study design, setting, and at-risk populations, the reported VE estimates against symptomatic mpox infection for one or two doses supports deployment of JYNNEOS for mpox outbreak control.
The U.S. Food and Drug Administration initially approved JYNNEOS for smallpox in September 2019 and extended its authorization for mpox in 2022. As of late 2023, about 1.2 million doses had been administered in 57 U.S. Jurisdictions.
As of May 2024, healthcare providers in the U.S. can order JYNNEOS through their preferred wholesaler and distribution partners to make it available for at-risk individuals at pharmacies.
According to a recent analysis, JYNNEOS booster (third) doses have not been recommended.
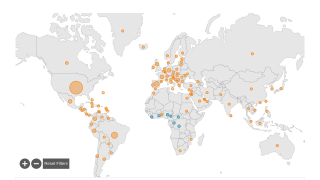
The World Health Organization recently published Mpox External Situation Report #32, which confirmed that from January 2022 through March 2024, a cumulative total of 95,226 mpox cases, including 185 deaths, were reported.
Note: Bavarian Nordic Inc., the owner of JYNNEOS, funded the research presented in this manuscript.
Our Trust Standards: Medical Advisory Committee